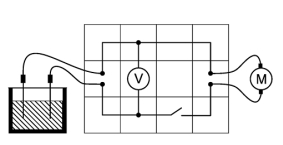
Principle
A galvanic element consists of two different metal electrodes which immerse into the aqueous solution of an electrolyte. An electrical voltage can be "tapped" between this electrodes, which can be easily explained by the fact that positive metal ions pass from the surface of the electrodes into the solution and free-moving electrons remain on the electrodes.
This experiment shows that an electrical voltage arises between two electrodes of different metals when they are connected in an aqueous electrolyte solution.
Benefits
- No additional cable connections between the building blocks needed - clear arragned and quick setup
- Contact saftey due to puzzle blocks system
- Corrosion-free gold plated contacts
- Doubled earning sucess: Electric circuit diagram on top, real components can be seen unterside