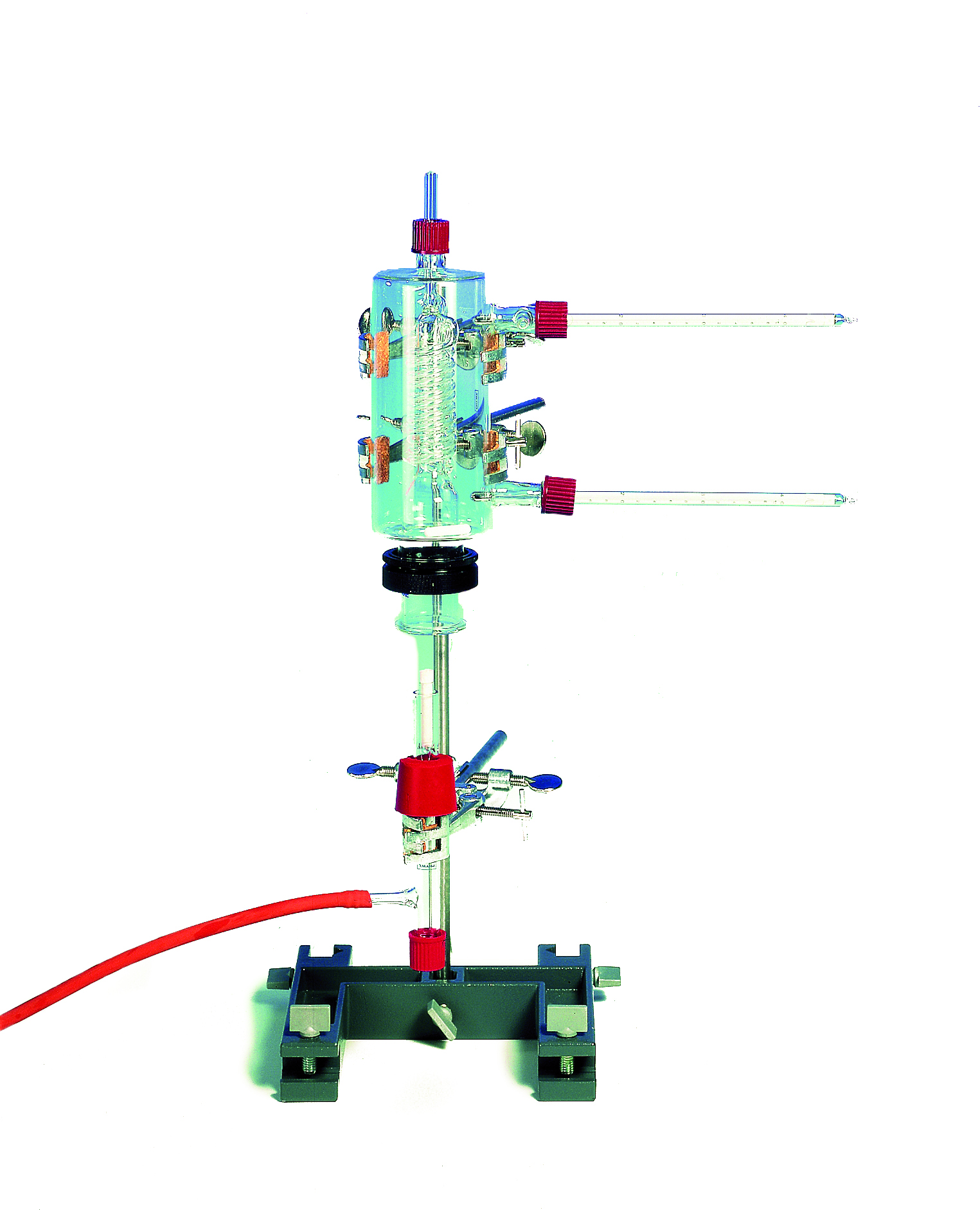
Principle
The heat of reaction generated during the complete combustion of 1000 g of solid or liquid fuel is known as the calorific value H. In the case of complete combustion of nutritional fats, the gross calorific value can also be determined. In order to ensure complete combustion, the reaction takes place under oxygen. The heat generated during the combustion of a specific amount of fuel is absorbed by a glass jacket calorimeter of known heat capacity. The calorific value of the test substance can be calculated from the temperature increase in the calorimeter.
Benefits
- No toxic or dangerous chemical is used for this experiment.
- Part of a system solution - glass jacket system easily expandable
Tasks
Determine the calorific value of heating oil and the gross calorific value of olive oil.
Learning objectives
- Heat of reaction
- Heat of combustion
- Enthalpy of combustion
- First law of thermodynamics
Necessary accessories
- Precision balance 620g/0.001g